Mice fed high-fat diet and treated with Gel-B (GS300 prototype) hydrogel were protected against the development of fatty liver and associated metabolic disorders
Preclinical data suggest Gel-B targets gut-related pathophysiologies that drive insulin resistance, NAFLD and NASH
BOSTON — Gelesis, a biotechnology company at the forefront of developing mechanobiology-based therapies to treat chronic diseases related to the gastrointestinal (GI) system, today announced compelling preclinical data suggesting that the Company’s proprietary hydrogel, Gel-B (GS300 prototype), prevents the harmful effects of a high-fat diet on the liver and associated metabolic disorders. The late-breaking poster was presented at the International Liver Congress 2019, held this week in Vienna, Austria.
Preclinical data presented by Gelesis last month at ENDO, The Endocrine Society Annual Meeting, demonstrated that Gel-B treatment, designed with specific mechanical properties, restored gut barrier function in mice after severe intestinal wall injury and prevented unwanted substances from reaching the circulatory system. Those findings suggested potential therapeutic utility for Gel-B in minimizing the low-grade systemic inflammation and diseases associated with gut barrier dysfunction.
The latest data presented today suggest that Gel-B not only has a protective effect on the gut barrier but may also work to prevent liver and metabolic disorders associated with high-fat diets. In the study, mice fed a high-fat diet and treated with Gel-B were protected from developing non-alcoholic fatty liver disease (NAFLD), insulin resistance and excessive weight gain.
“There is a rising epidemic of non-alcoholic fatty liver disease worldwide and a desperate need for therapeutic agents. These data suggest a unique approach to treat fatty liver diseases by restoring the normal function of the gut barrier and warrant further investigation,” said Arun Sanyal, M.D., a gastroenterologist at the Virginia Commonwealth University School of Medicine and advisor to the Company. “This research demonstrates an improvement in GLP-1 levels, change in gut flora and upregulation of tight junctions, all of which could improve the integrity of the gut wall and prevent fat accumulation and other harmful substances from reaching and inflaming the liver.”
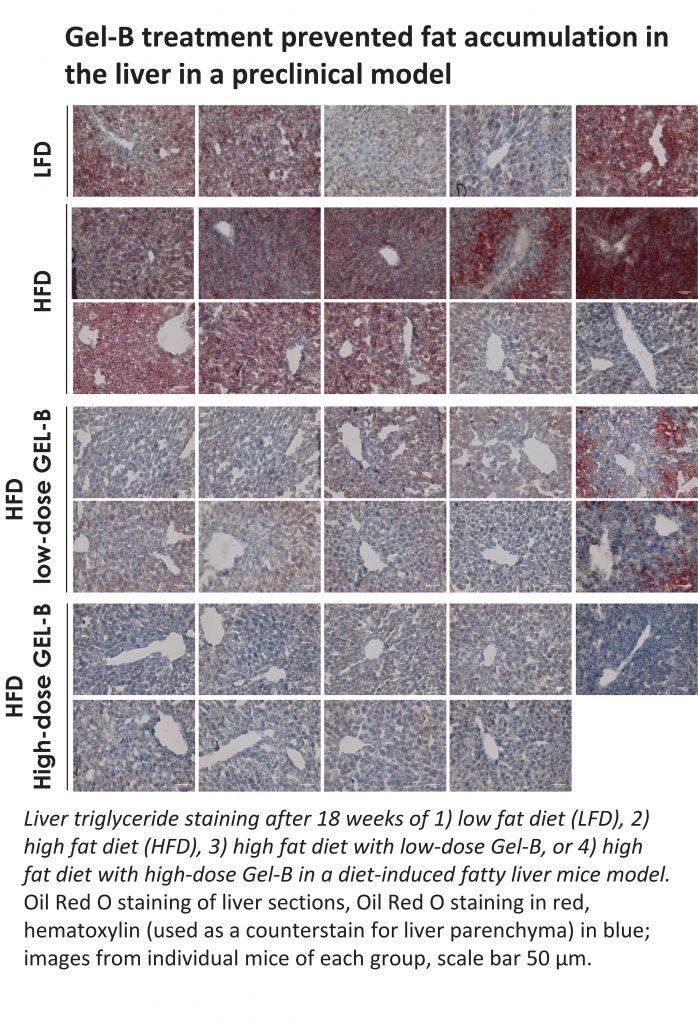
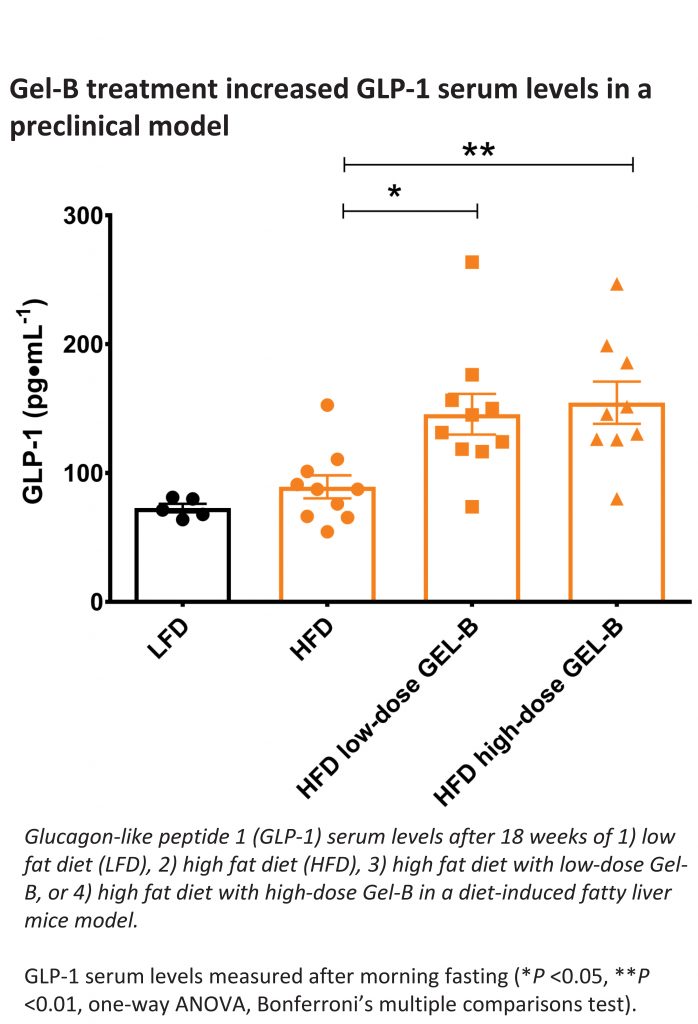
In the study presented at EASL, mice were divided into four groups: low-fat diet, high-fat diet and high-fat diet enhanced with either low or high doses of Gel-B, a proprietary non-systemic hydrogel previously shown to restore gut barrier function in a preclinical model. Gel-B prevented the development of fatty liver in all mice in the high dose group and most of the mice on the low dose, despite being fed with a high fat diet (45% lard) for 18 weeks. Those treated with Gel-B along with the high-fat diet also showed significant improvements across multiple measures of metabolic function as compared with the high-fat control (mice on the same diet without Gel-B). Among the observed changes: mice on Gel-B treatment had a significant reduction of insulin resistance as measured by homeostatic model assessment – insulin resistance (HOMA-IR), decreased insulin levels and higher levels of GLP-1, a gut hormone known to affect liver health.
“We’re excited to share new insights into the effects of this new investigational approach on the gut barrier. Preclinical studies indicate that Gel-B protects against the negative effects of a high-fat diet by enhancing the intestinal barrier properties through a mechanical action leading to closing the gaps between intestinal epithelial cells, which make up the first layer of defense in the gut,” said Elaine Chiquette, Pharm.D., Chief Scientific Officer at Gelesis. “We continue to collaborate with key researchers to understand additional mechanisms of action, such as changes in microbiome and gut hormones, to advance our understanding of Gel-B effects on gut and liver health.”
Gelesis’ proprietary hydrogels are orally administered and made from two naturally derived building blocks – modified cellulose cross-linked with citric acid – that create a three-dimensional matrix to achieve specific mechanical properties through the GI.
Gelesis is developing a novel hydrogel platform technology to treat overweight, obesity and chronic diseases related to the GI pathway. Gelesis’ proprietary approach is designed to act mechanically in the GI pathway to potentially alter the course of chronic diseases. The company’s lead product candidate, Gelesis100, has been submitted to the FDA for review as an aid to weight management. Additionally, Gelesis is developing its second candidate, Gelesis200, a hydrogel optimized for weight loss and glycemic control in patients with type 2 diabetes and prediabetes. Novel hydrogel mechanotherapeutics based on the Gelesis platform technology are also being advanced through a pipeline in other GI inflammatory conditions where gut barrier and gut permeability potentially play a role, such as non-alcoholic steatohepatitis (NASH) and inflammatory bowel disease (IBD).
The Gelesis executive and advisory team includes some of the world’s leading experts in obesity, materials science, chronic disease research and commercialization. Gelesis was co-founded by PureTech Health (LSE: PRTC), a biopharmaceutical company focused on the Brain-Immune-Gut (BIG) Axis. For more information, visit gelesis.com or connect with us on Twitter @GelesisInc.
Contacts
Kathryn McNeil
+1 347 204 4226
kmcneil@gelesis.com